Exhibit 99.1
JP Morgan January 2017
Exhibit 99.1
JP Morgan January 2017

Disclaimer This presentation contains “forward-looking statements,” as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as “believe,” “may”, “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect” and other words of similar meaning. These forward-looking statements involve certain risks and uncertainties. Such risks and uncertainties could cause our actual results to differ materially from those indicated by such forward-looking statements, and include, without limitation: the success, cost and timing of our product development activities and clinical trials; our ability to submit an IND and successfully advance our technology platform to improve the safety and effectiveness of our existing TCR therapeutic candidates; the rate and degree of market acceptance of T-cell therapy generally and of our TCR therapeutic candidates; government regulation and approval, including, but not limited to, the expected regulatory approval timelines for TCR therapeutic candidates; and our ability to protect our proprietary technology and enforce our intellectual property rights; amongst others. For a further description of the risks and uncertainties that could cause our actual results to differ materially from those expressed in these forward-looking statements, as well as risks relating to our business in general, we refer you to our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on November 10, 2016 and our other SEC filings. We urge you to consider these factors carefully in evaluating the forward-looking statements herein and are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. The forward-looking statements contained in this presentation speak only as of the date the statements were made and we do not undertake any obligation to update such forward-looking statements to reflect subsequent events or circumstances. We intend that all forward-looking statements be subject to the safe-harbor provisions of the PSLRA. Adaptimmune® and SPEAR® are registered trademarks of Adaptimmune. 2
Adaptimmune: Leading the TCR T-cell Space Pioneering Peerless Industry leading Transformative Data driven Forward-thinking 24 years of TCR innovation The only affinity- tuned TCRs Unmatched SPEAR® T-cell pipeline Proven efficacy in solid tumors Informed approach to clinical development Combination and next generation approaches 3
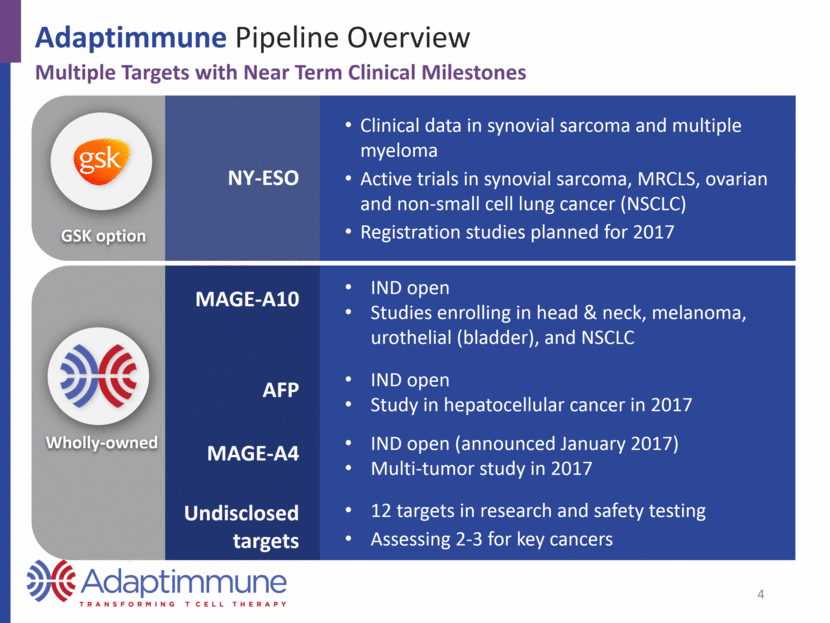
Adaptimmune Pipeline Overview Multiple Targets with Near Term Clinical Milestones Clinical data in synovial sarcoma and multiple myeloma Active trials in synovial sarcoma, MRCLS, ovarian and non-small cell lung cancer (NSCLC) Registration studies planned for 2017 NY-ESO IND open Studies enrolling in head & neck, melanoma, urothelial (bladder), and NSCLC IND open Study in hepatocellular cancer in 2017 IND open (announced January 2017) Multi-tumor study in 2017 12 targets in research and safety testing Assessing 2-3 for key cancers MAGE-A10 AFP MAGE-A4 Undisclosed targets GSK option Wholly-owned 4
Unmatched Clinical Pipeline of Affinity Enhanced TCRs SPEAR target Indication Notes Pre-IND Phase I / II Registration trial NY-ESO Synovial sarcoma Registration trial Cohort 1 - High NY-ESO + CTX / FLU Cohort 2 - Low NY-ESO + CTX / FLU Cohort 3 – no FLU Cohort 4 – modified CTX / FLU Myxoid / Round cell liposarcoma Pilot study Multiple myeloma Autologous SCT Combination with anti-PD1 (KEYTRUDA) Ovarian No FLU Modified CTX / FLU Melanoma No Flu Non-small cell lung cancer (NSCLC) Modified CTX / FLU MAGE-A10 NSCLC Modified CTX / FLU Urothelial (bladder), melanoma, H&N Modified CTX / FLU AFP Hepatocellular cancer Modified CTX / FLU MAGE-A4 Urothelial, melanoma, H&N, ovarian, NSCLC, esophageal, gastric Planned Complete Ongoing 5
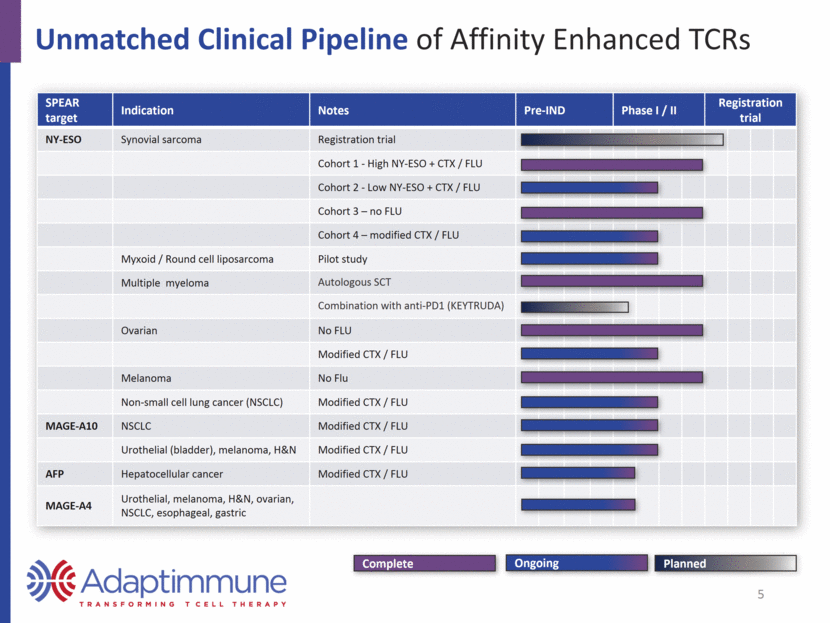
2017: A Year of Significant Data Delivery Potential for Data from Multiple SPEAR T-cell Therapies in Multiple Tumors NY-ESO Synovial Sarcoma Cohorts 1, 2, and 4 MRCLS Multiple Myeloma + KEYTRUDA Ovarian + Flu NSCLC MAGE-A10 NSCLC Urothelial (bladder), melanoma, H&N MAGE-A4 Urothelial, melanoma, H&N, ovarian, NSCLC, esophageal, and gastric AFP Hepatocellular 6
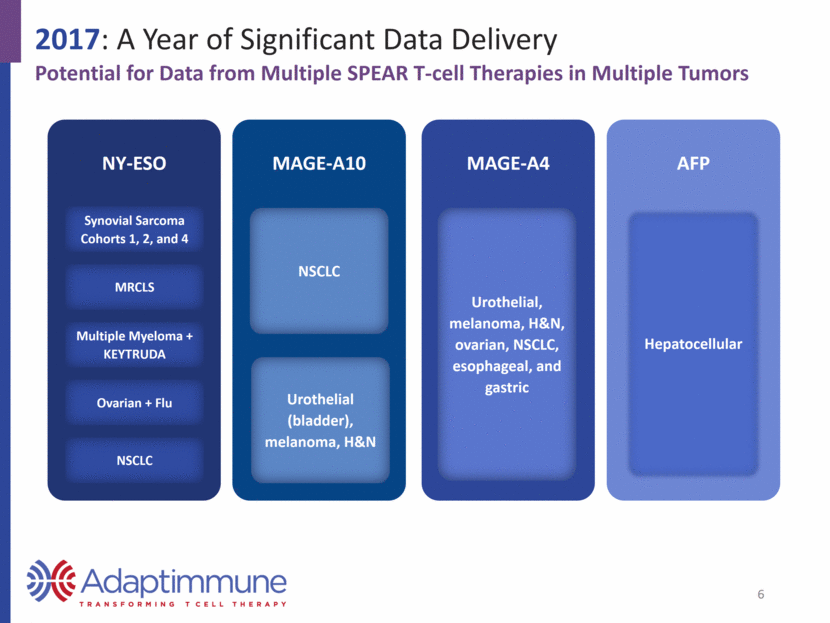
Patient Outcomes Depend on Great Science Innovation Drives Patient Care Technology Foundation Identifies the right targets TCR generation against virtually any HLA/target Affinity enhancement Proprietary preclinical safety platform Manufacturing Technology Clinical Foundation Treatment / Post-treatment Continuous Evolution Robust process for T-cell manufacturing Proprietary vector manufacturing process Exclusive license for CD3/CD28 Dynabeads® Alliances with major cancer centers Optimal preconditioning Optimal in vivo expansion, long-term antitumor memory, SPEAR T-cell persistence Optimal cell dose; CD4 and CD8 cells Potential to re-treat Duration of response Persistence without exhaustion Mechanisms of resistance and relapse Enhancements to impact immune suppression Assessment of combination therapies Allogeneic approaches 7
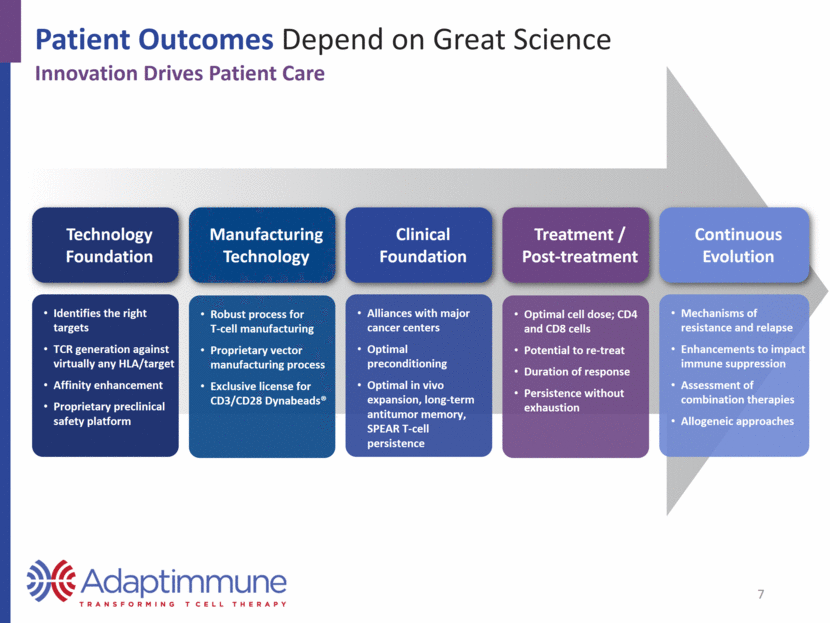
Nearly all proteins are available to TCRs Unlimited targets; utilizes the T-cell’s native receptor Affinity tuned SPEAR TCRs overcome low target expression; required to address solid tumors Access to extra- and intracellular proteins CAR-T vs TCR: Differences in Access to Human Proteome TCRs CAR-T Significantly Better Access to Peptides with T-cell Receptors Only ~28% of proteins available to CAR-T cells Chimeric antigen receptor; not designed to recognize an HLA peptide Mostly limited to extracellular proteins Limited targets compared to TCRs 8
Affinity Optimization is Critical to Address Majority of Antigens Adaptimmune is the Only Company with this Proprietary Technology T-cells bind to targets on cancer cells Cancer downregulates targets to avoid detection Most naturally occurring anti-tumor T-cells are low affinity (require more targets) SPEAR T-cells are affinity enhanced to overcome this problem Proprietary preclinical engineering ensures tumor-specific response Optimal specificity and affinity for antitumor activity Demonstrated efficacy in solid tumors 9
Clinical Data Overview 10

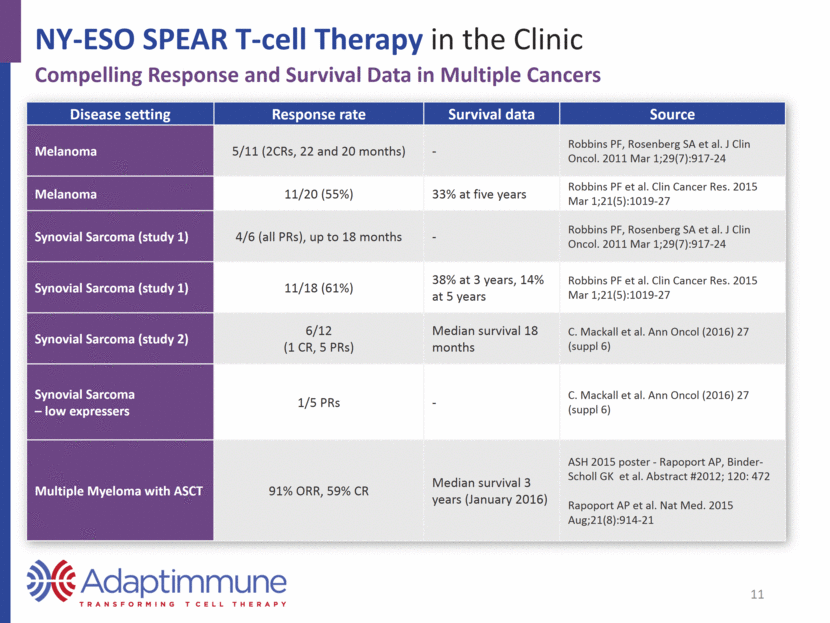
NY-ESO SPEAR T-cell Therapy in the Clinic Compelling Response and Survival Data in Multiple Cancers Disease setting Response rate Survival data Source Melanoma 5/11 (2CRs, 22 and 20 months) - Robbins PF, Rosenberg SA et al. J Clin Oncol. 2011 Mar 1;29(7):917-24 Melanoma 11/20 (55%) 33% at five years Robbins PF et al. Clin Cancer Res. 2015 Mar 1;21(5):1019-27 Synovial Sarcoma (study 1) 4/6 (all PRs), up to 18 months - Robbins PF, Rosenberg SA et al. J Clin Oncol. 2011 Mar 1;29(7):917-24 Synovial Sarcoma (study 1) 11/18 (61%) 38% at 3 years, 14% at 5 years Robbins PF et al. Clin Cancer Res. 2015 Mar 1;21(5):1019-27 Synovial Sarcoma (study 2) 6/12 (1 CR, 5 PRs) Median survival 18 months C. Mackall et al. Ann Oncol (2016) 27 (suppl 6) Synovial Sarcoma – low expressers 1/5 PRs - C. Mackall et al. Ann Oncol (2016) 27 (suppl 6) Multiple Myeloma with ASCT 91% ORR, 59% CR Median survival 3 years (January 2016) ASH 2015 poster - Rapoport AP, Binder-Scholl GK et al. Abstract #2012; 120: 472 Rapoport AP et al. Nat Med. 2015 Aug;21(8):914-21 11
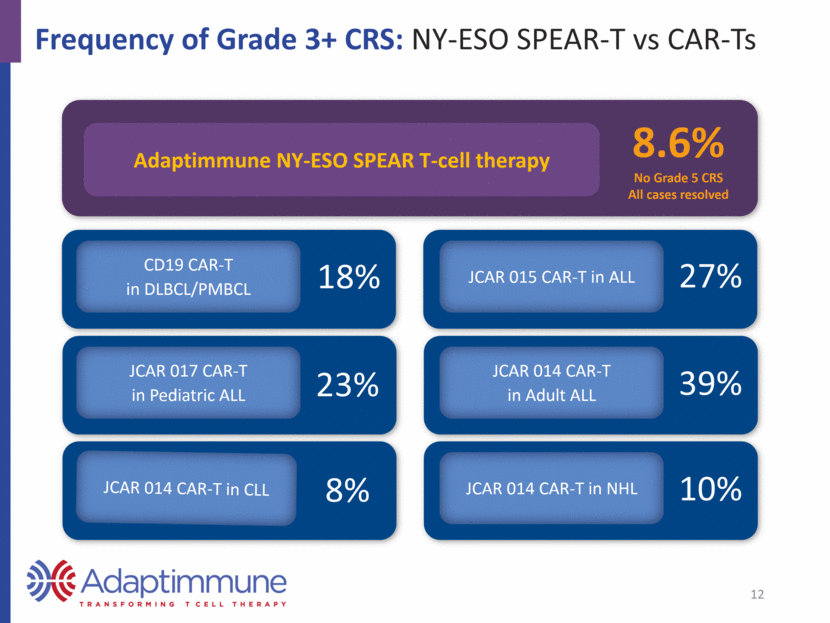
Frequency of Grade 3+ CRS: NY-ESO SPEAR-T vs CAR-Ts Adaptimmune NY-ESO SPEAR T-cell therapy 8.6% No Grade 5 CRS All cases resolved 18% CD19 CAR-T in DLBCL/PMBCL 27% 23% 39% JCAR 017 CAR-T in Pediatric ALL JCAR 014 CAR-T in Adult ALL JCAR 015 CAR-T in ALL 8% 10% JCAR 014 CAR-T in CLL JCAR 014 CAR-T in NHL 12
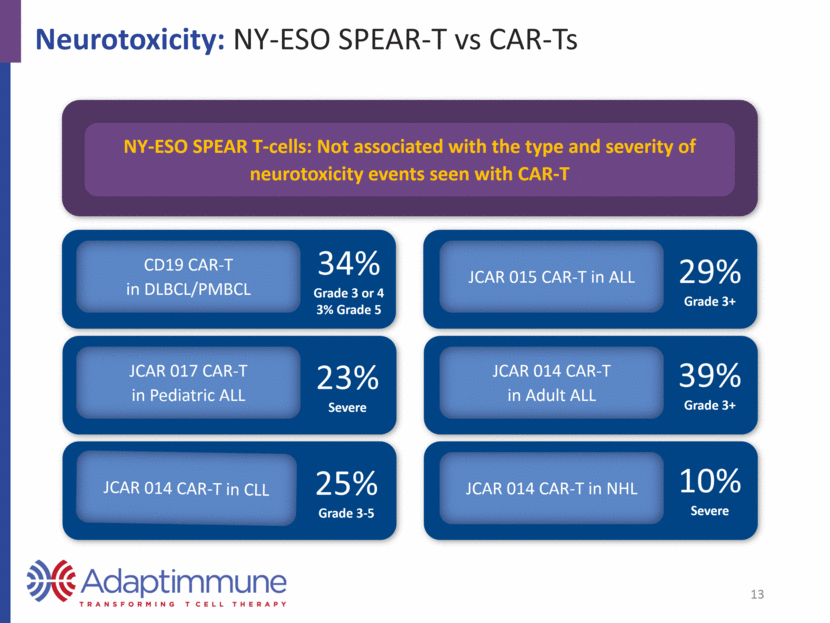
Neurotoxicity: NY-ESO SPEAR-T vs CAR-Ts NY-ESO SPEAR T-cells: Not associated with the type and severity of neurotoxicity events seen with CAR-T 34% Grade 3 or 4 3% Grade 5 CD19 CAR-T in DLBCL/PMBCL 23% Severe JCAR 017 CAR-T in Pediatric ALL JCAR 014 CAR-T in Adult ALL JCAR 015 CAR-T in ALL JCAR 014 CAR-T in CLL JCAR 014 CAR-T in NHL 25% Grade 3-5 29% Grade 3+ 39% Grade 3+ 10% Severe 13
Adaptimmune’s Technology Platform 14
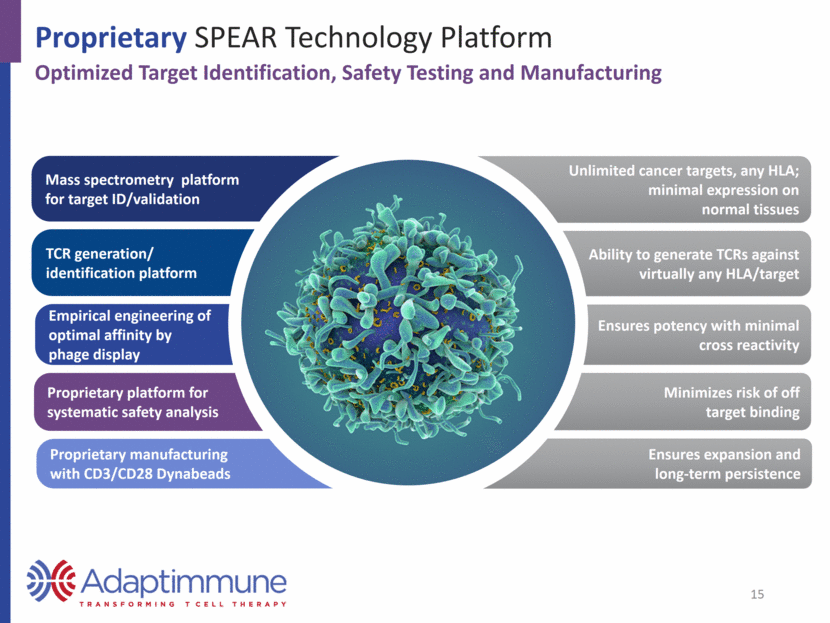
Ability to generate TCRs against virtually any HLA/target Proprietary SPEAR Technology Platform Mass spectrometry platform for target ID/validation TCR generation/ identification platform Empirical engineering of optimal affinity by phage display Proprietary platform for systematic safety analysis Unlimited cancer targets, any HLA; minimal expression on normal tissues Ensures potency with minimal cross reactivity Minimizes risk of off target binding Optimized Target Identification, Safety Testing and Manufacturing 15 Proprietary manufacturing with CD3/CD28 Dynabeads Ensures expansion and long-term persistence
Prioritizing Targets for Clinical Assessment 16

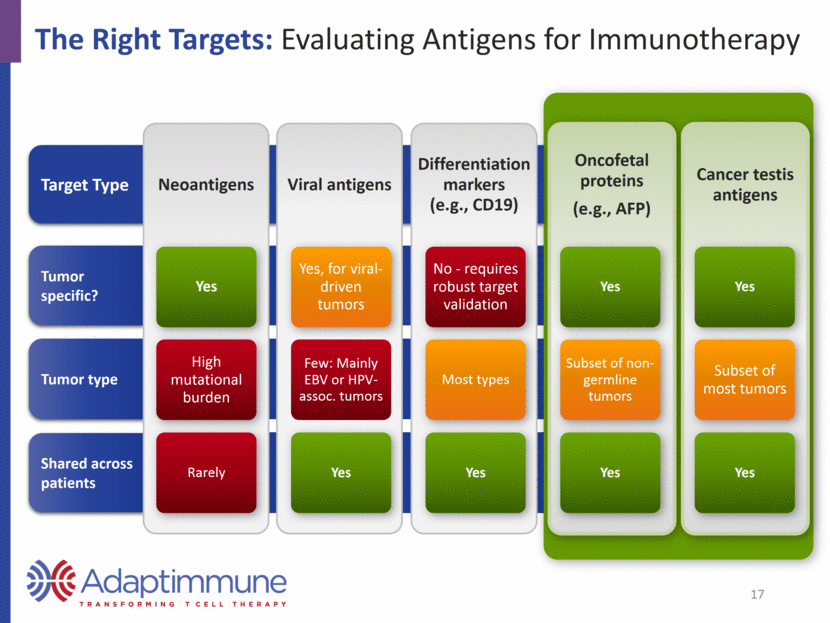
The Right Targets: Evaluating Antigens for Immunotherapy Shared across patients Tumor type Tumor specific? Target Type 17 Neoantigens Yes High mutational burden Rarely Viral antigens Yes, for viral-driven tumors Few: Mainly EBV or HPV-assoc. tumors Yes Differentiation markers (e.g., CD19) No - requires robust target validation Most types Yes Oncofetal proteins (e.g., AFP) Yes Subset of non-germline tumors Yes Cancer testis antigens Yes Subset of most tumors Yes
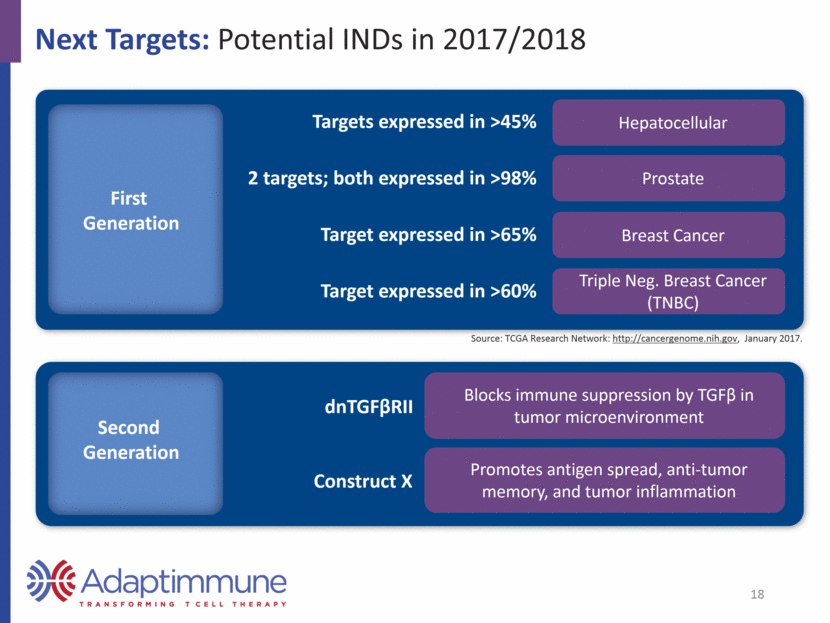
Next Targets: Potential INDs in 2017/2018 Prostate Breast Cancer Triple Neg. Breast Cancer (TNBC) 2 targets; both expressed in >98% Target expressed in >60% Target expressed in >65% Targets expressed in >45% Hepatocellular First Generation Promotes antigen spread, anti-tumor memory, and tumor inflammation Blocks immune suppression by TGFβ in tumor microenvironment Construct X dnTGFβRII Second Generation 18 Source: TCGA Research Network: http://cancergenome.nih.gov, January 2017.
Prioritizing Cancers with Significant Unmet Medical Need1 AML Prostate Pancreatic Colon SCLC Breast Gastric NSCLC (Adeno and Squamous) 19,950 new cases 10,430 deaths 180,890 new cases 26,120 deaths 53,070 new cases 41,780 deaths 134,490 new cases 49,190 deaths 33,656 new cases 23,712 deaths2 246,660 new cases 40,450 deaths 26,370 new cases 10,730 deaths 190,732 new cases 134,368 deaths3 Prioritizing targets expressed by up to 99% of: 1 American Cancer Society 2016 estimates 2 ACS: Approximately 15% of all lung cancers are SCLC 3 ACS: Approximately 85% of all lung cancers are NSCLC 19
Adaptimmune Pipeline 20
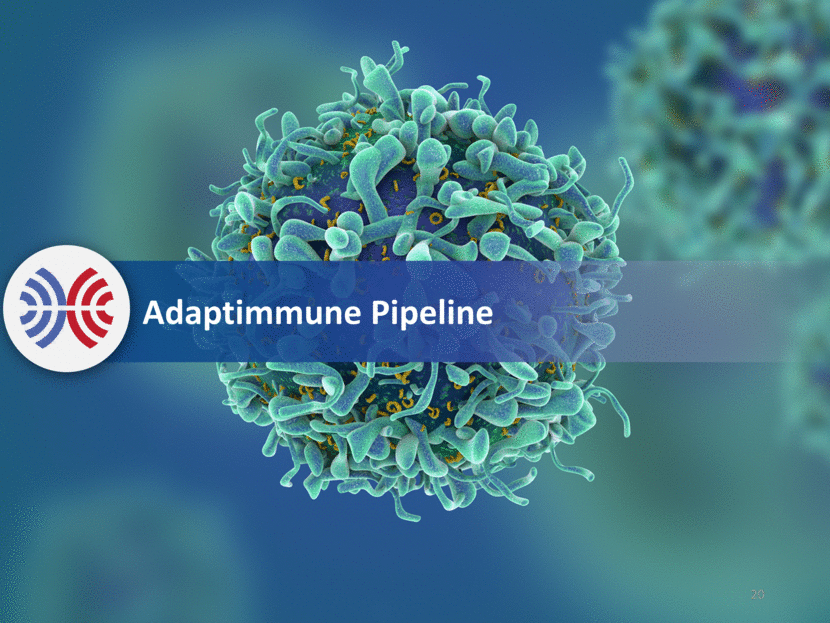
Deep Pipeline Across Major Cancers NY-ESO: Expressed Across a Wide Range of Tumors Estimated Annual Deaths Source: seer.cancer.gov; http://www.cancer.org/; 2016 data Source: eco.iarc.fr/eucan; 2012 data US1 Europe2 Soft tissue sarcoma 4,990 - Myeloma 12,650 24,287 Ovarian 14,240 42,716 Melanoma 10,130 22,199 Lung 158,080 353,580 21 NY-ESO Expression Source: TCGA Research Network: http://cancergenome.nih.gov, January 2017. 0 20 40 60 80 100 Positive tumors (%)
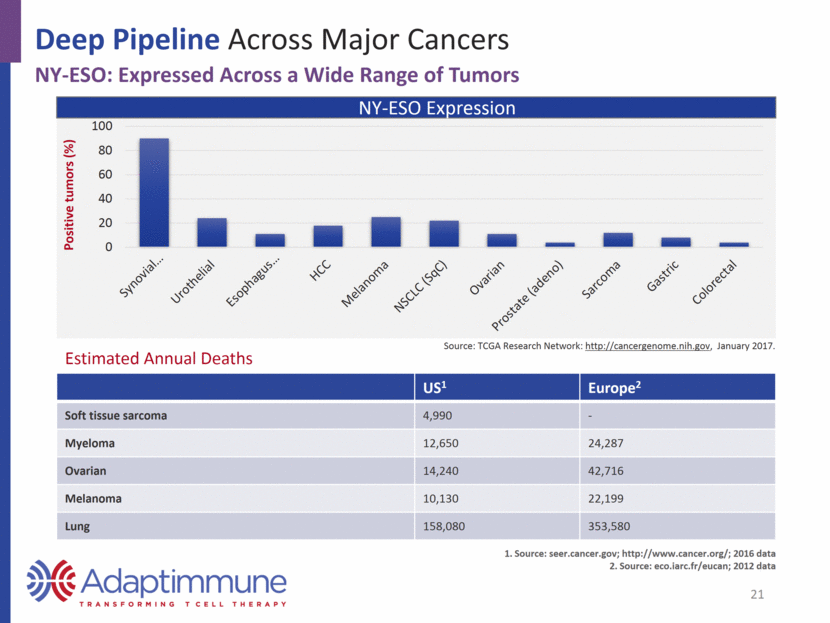
Deep Pipeline Across Major Cancers NY-ESO SPEAR T-cell Development Program: Sarcoma SPEAR target Indication Notes Pre-IND Phase I / II Registration NY-ESO Synovial sarcoma Registration Cohort 1 - High NY-ESO +CTX / FLU Cohort 2 - Low NY-ESO +CTX / FLU Cohort 3 – no fludarabine Cohort 4 – modified CTX / FLU Myxoid / Round cell liposarcoma Pilot study NY-ESO SPEAR T-cells in Synovial Sarcoma ~18 months (80 weeks) median survival for cohort 1 60% response rate (6/10) in patients receiving target cell dose (50% overall response rate [6/12]) in context of CTX + fludarabine Confirmed response seen in 1 of 5 patients with low NY-ESO expression Overall, manageable toxicity; highly persistent cells in the presence of fludarabine 2017 Milestones: Data from synovial sarcoma cohorts 1, 2, and 4; MRCLS pilot study 22 Planned Complete Ongoing
2017 Milestones: Initiation of combination study with KEYTRUDA®; potential for data in late 2017 Deep Pipeline Across Major Cancers NY-ESO SPEAR T-cell Development Program: Multiple Myeloma SPEAR target Indication Notes Pre-IND Phase I / II Registration NY-ESO Multiple myeloma Autologous SCT Combination with anti-PD1 (KEYTRUDA) NY-ESO SPEAR T-cells in Multiple Myeloma 3-year overall survival (OS) as of Jan. 2016 91 percent (20/22) response rate at day 100 Median: PFS=19.1 months (11/2015) Manageable toxicity, highly persistent cells 23 Planned Complete Ongoing
2017 Milestones: Data from studies in NSCLC and ovarian (with FLU) Deep Pipeline Across Major Cancers NY-ESO SPEAR T-cell Development Programs: Ovarian, Melanoma, and NSCLC Results of ovarian and melanoma studies with CTX only highlight need for preconditioning regimen including fludarabine SPEAR target Indication Notes Pre-IND Phase I / II Registration NY-ESO Ovarian No fludarabine modified CTX / FLU Melanoma No fludarabine Non-small cell lung cancer (NSCLC) modified CTX / FLU 24 Planned Complete Ongoing
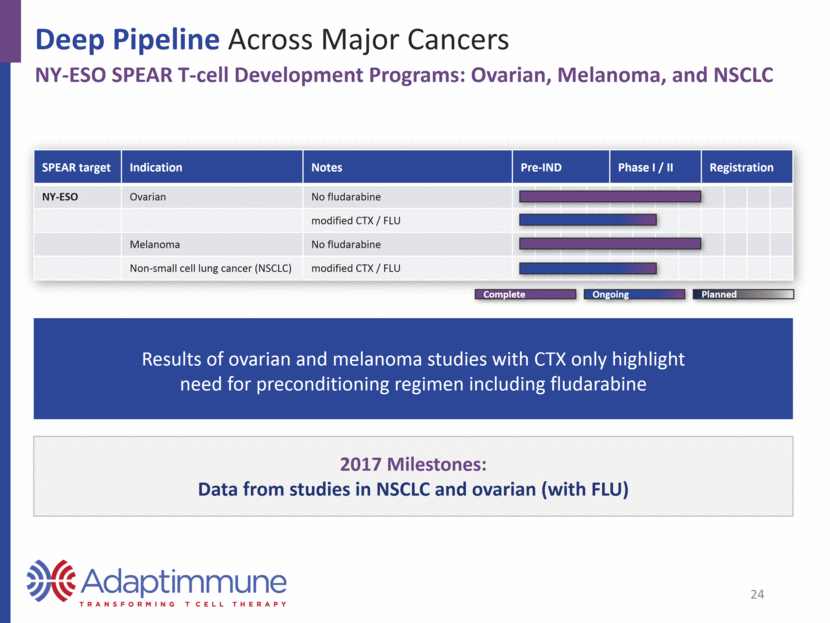
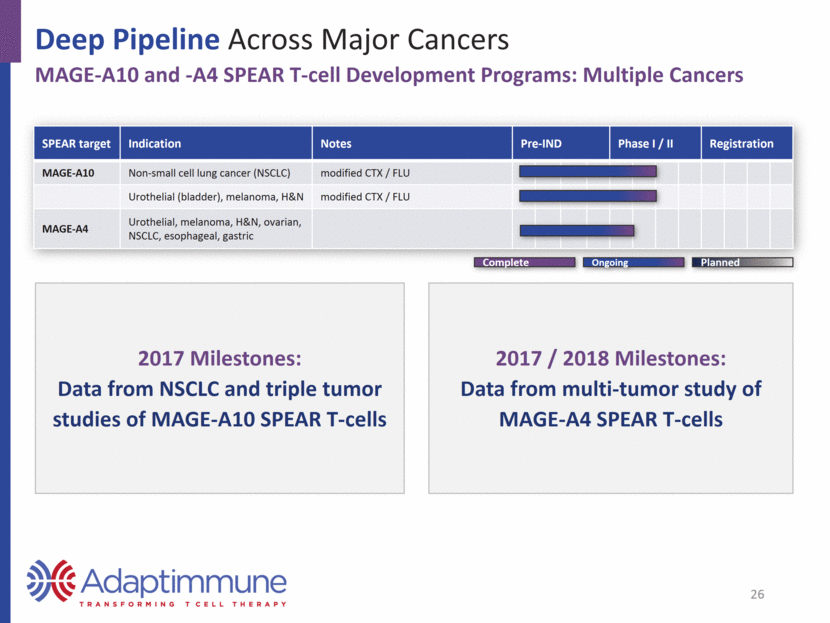
Deep Pipeline Across Major Cancers MAGE-A10 and -A4: Expressed Across a Wide Range of Tumors MAGE-A10 Expression MAGE-A4 Expression US1 Europe2 Urothelial 16,390 52,374 Head and neck 9,570 43,704 Ovarian 14,240 42,716 Melanoma 10,130 22,199 Lung 158,080 353,580 Esophageal 15,690 39,504 Gastric 10,730 107,313 Source: seer.cancer.gov; http://www.cancer.org/; 2016 data Source: eco.iarc.fr/eucan; 2012 data Estimated Annual Deaths 25 Source: TCGA Research Network: http://cancergenome.nih.gov, January 2017. 0 20 40 60 80 100 Positive tumors (%) 0 20 40 60 80 100 Positive tumors (%)
Deep Pipeline Across Major Cancers MAGE-A10 and -A4 SPEAR T-cell Development Programs: Multiple Cancers SPEAR target Indication Notes Pre-IND Phase I / II Registration MAGE-A10 Non-small cell lung cancer (NSCLC) modified CTX / FLU Urothelial (bladder), melanoma, H&N modified CTX / FLU MAGE-A4 Urothelial, melanoma, H&N, ovarian, NSCLC, esophageal, gastric 2017 Milestones: Data from NSCLC and triple tumor studies of MAGE-A10 SPEAR T-cells 2017 / 2018 Milestones: Data from multi-tumor study of MAGE-A4 SPEAR T-cells 26 Planned Complete Ongoing
Deep Pipeline Across Major Cancers Building a Franchise: Broad Coverage of Cancers with Existing CTA Pipeline Lung Squamous Cell NY-ESO-1 22% MAGE-A10 33% MAGE-A4 60% Expression by 1 or more 65% Head & Neck Cancer (squamous cell) NY-ESO-1 10% MAGE-A10 14% MAGE-A4 42% Expression by 1 or more 44% Tumor Overlap Examples 27 Urothelial Cancer NY-ESO-1 24% MAGE-A10 31% MAGE-A4 35% Expression by 1 or more 48% Source: TCGA Research Network: http://cancergenome.nih.gov, January 2017.
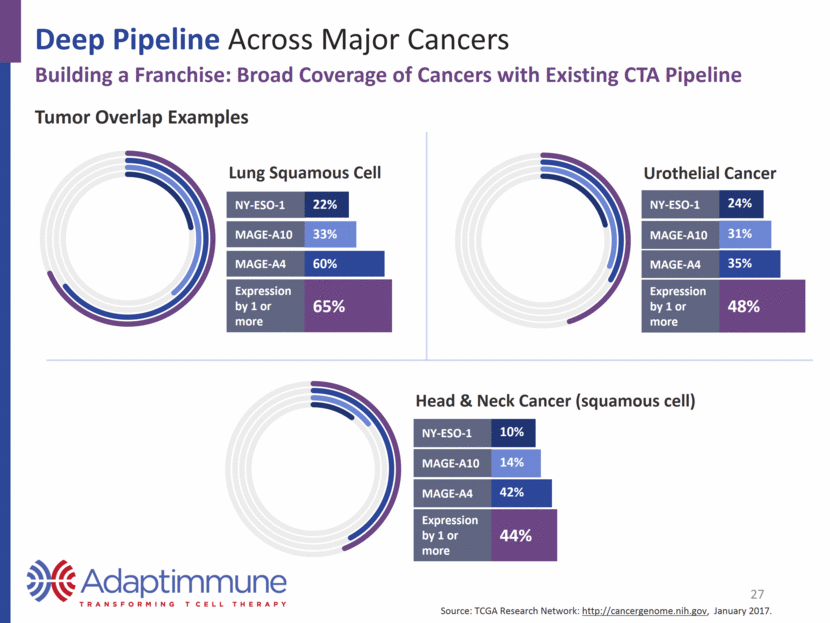
Deep Pipeline Across Major Cancers AFP SPEAR T-cell Development Program: Hepatocellular cancer SPEAR target Indication Notes Pre-IND Phase I / II Registration AFP Hepatocellular cancer Modified CTX / FLU AFP Expression US1 Europe2 Liver HCC 27,170 62,152 2017/2018 Milestones: Data from study in hepatocellular cancer Source: seer.cancer.gov; http://www.cancer.org/; 2016 data Source: eco.iarc.fr/eucan; 2012 data Estimated Annual Deaths 28 Planned Complete Ongoing Source: TCGA Research Network: http://cancergenome.nih.gov, January 2016. 0 20 40 60 80 100 Liver HCC Positive tumors (%)
Leading Innovation in Engineered T-cell Therapy Next Generation: Depth and Durability in Solid Tumors Combination studies starting in 2017 Enhancing resistance to tumor microenvironment: 5 programs and growing Enhancing T-cell potency and function: 11 programs and growing Enhancement of Class-I restricted CD4 T-cell function Enhancement of cytotoxic function Enhancement of epitope spreading Other internal programs in development Partnership with Bellicum 29 Block effects of immunosuppression (e.g., TGF-β) Overcoming metabolic restrictions of tumor environment Other internal programs in development
Leading Innovation in Engineered T-cell Therapy Innovative Partnership with Bellicum Staged collaboration to evaluate Bellicum’s “GoTCR” switch technology Technology could complement our next generation efforts Provides potential on/off switch to T-cell May further enhance SPEAR T-cell proliferation, activation and persistence Preclinical POC will be completed in 2017 Potential to proceed into co-development / co-commercialization phase in 2017/2018 30
Leading Innovation in Engineered T-cell Therapy Allogeneic Approach to TCR T-cell Therapy Partnered with Universal Cells Benefits of allogeneic approach include Progenitor cell line evaluated; T-cell differentiation ongoing Pre-IND meeting in planning Allows one manufacturing batch to treat numerous patients Enhanced control and standardization of manufactured product Eliminates risk of rejection by host and GvHD Decreases manufacturing costs Scalable for unlimited commercial manufacture 31
Optimizing T-cell Product Manufacturing 32

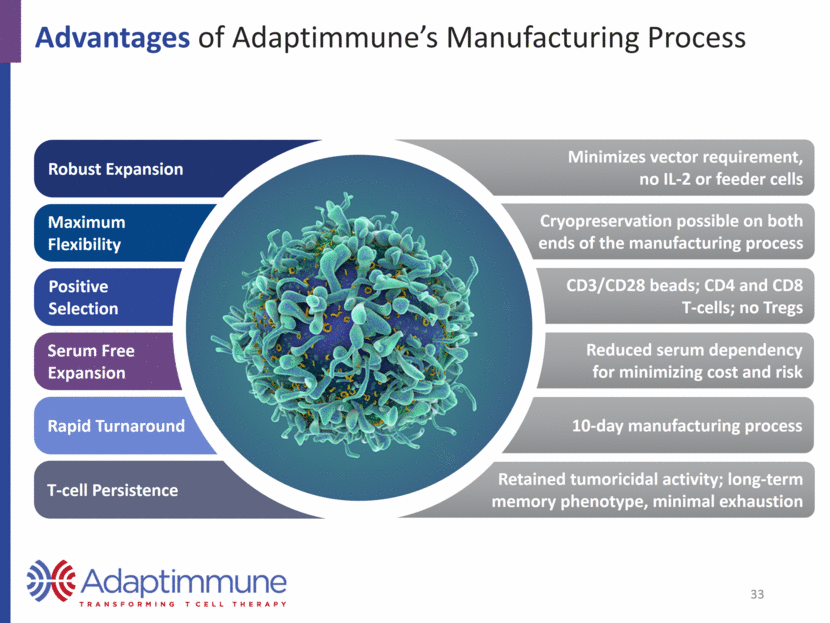
Advantages of Adaptimmune’s Manufacturing Process Robust Expansion Maximum Flexibility Positive Selection Serum Free Expansion Rapid Turnaround T-cell Persistence Minimizes vector requirement, no IL-2 or feeder cells Cryopreservation possible on both ends of the manufacturing process CD3/CD28 beads; CD4 and CD8 T-cells; no Tregs Reduced serum dependency for minimizing cost and risk 10-day manufacturing process Retained tumoricidal activity; long-term memory phenotype, minimal exhaustion 33
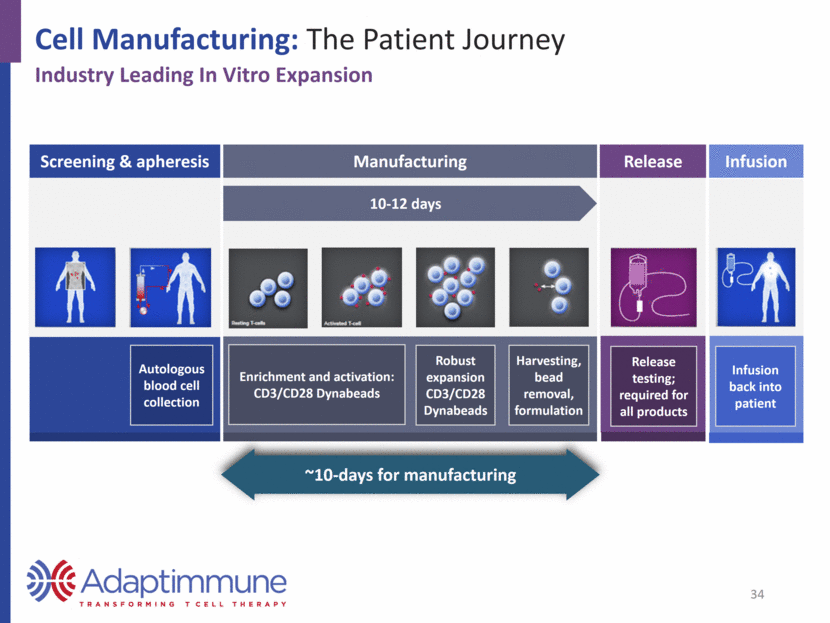
Cell Manufacturing: The Patient Journey Industry Leading In Vitro Expansion Screening & apheresis Manufacturing Release Infusion 10-12 days Autologous blood cell collection Enrichment and activation: CD3/CD28 Dynabeads Robust expansion CD3/CD28 Dynabeads Harvesting, bead removal, formulation Release testing; required for all products Infusion back into patient ~10-days for manufacturing 34
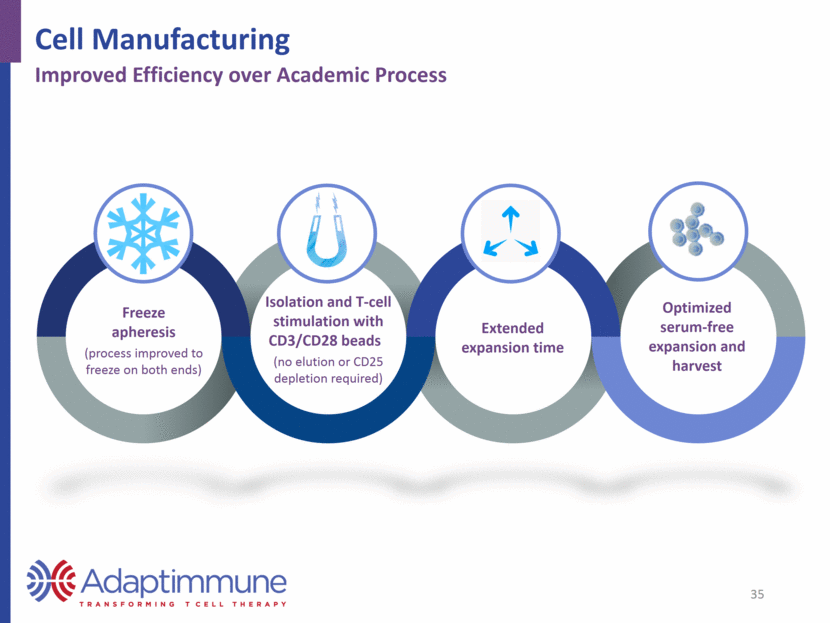
Freeze apheresis (process improved to freeze on both ends) Isolation and T-cell stimulation with CD3/CD28 beads (no elution or CD25 depletion required) Extended expansion time Optimized serum-free expansion and harvest 35 Cell Manufacturing Improved Efficiency over Academic Process
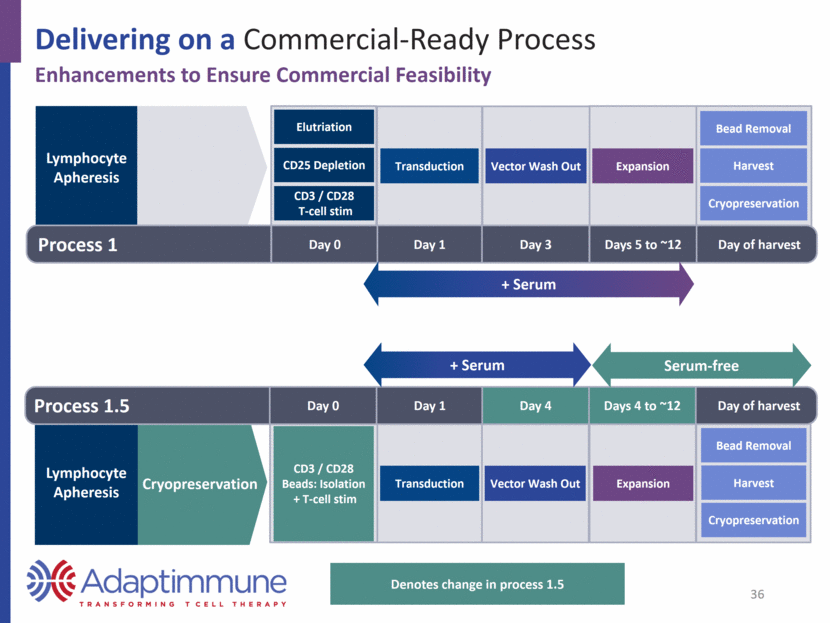
Delivering on a Commercial-Ready Process Enhancements to Ensure Commercial Feasibility Lymphocyte Apheresis Lymphocyte Apheresis Process 1 Day 0 Day of harvest Day 1 Day 3 Days 5 to ~12 Elutriation CD25 Depletion CD3 / CD28 T-cell stim Transduction Vector Wash Out Expansion Bead Removal Harvest Cryopreservation CD3 / CD28 Beads: Isolation + T-cell stim Cryopreservation Transduction Vector Wash Out Expansion Bead Removal Harvest Cryopreservation Denotes change in process 1.5 Process 1.5 Day 0 Day of harvest Day 1 Day 4 Days 4 to ~12 + Serum + Serum Serum-free 36
Corporate Update 37

Global Technology Network: Partnering with Industry Leaders Manufacturing Platform development Clinical 38
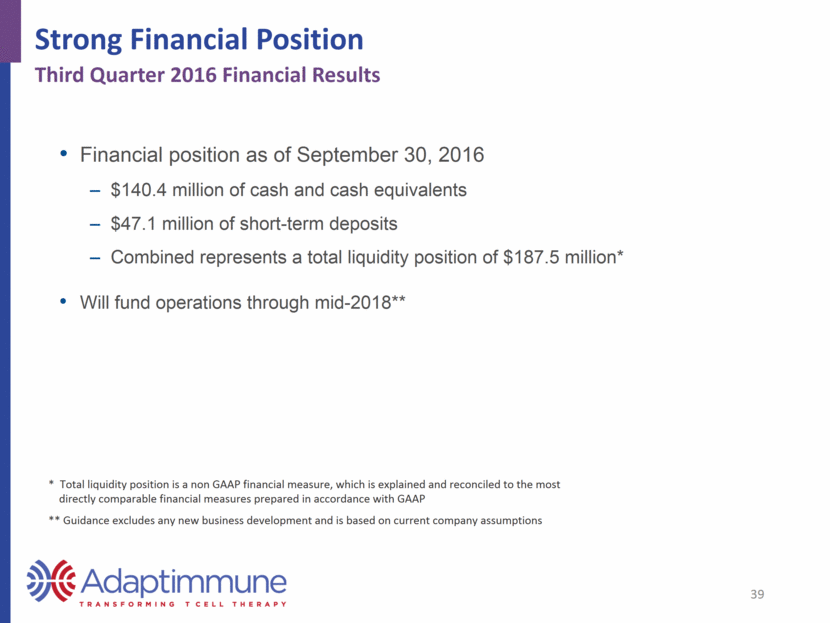
Strong Financial Position * Total liquidity position is a non GAAP financial measure, which is explained and reconciled to the most directly comparable financial measures prepared in accordance with GAAP ** Guidance excludes any new business development and is based on current company assumptions Financial position as of September 30, 2016 $140.4 million of cash and cash equivalents $47.1 million of short-term deposits Combined represents a total liquidity position of $187.5 million* Will fund operations through mid-2018** 39 Third Quarter 2016 Financial Results
JP Morgan January 2017
